Led by the Head and Neck Group of the Instituto Universitario de Oncología del Principado de Asturias (IUOPA), University of Oviedo.
In Cancers
http://www.mdpi.com/2072-6694/10/9/334, https://www.ncbi.nlm.nih.gov/pubmed/30227608
Cancers (Basel). 2018 Sep 17;10(9). pii: E334. doi: 10.3390/cancers10090334.
Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets.
Álvarez-Teijeiro S1,2, García-Inclán C3, Villaronga MÁ4,5, Casado P6, Hermida-Prado F7, Granda-Díaz R8, Rodrigo JP9,10, Calvo F11, Del-Río-Ibisate N12, Gandarillas A13, Morís F14, Hermsen M15,16, Cutillas P17, García-Pedrero JM18,19.
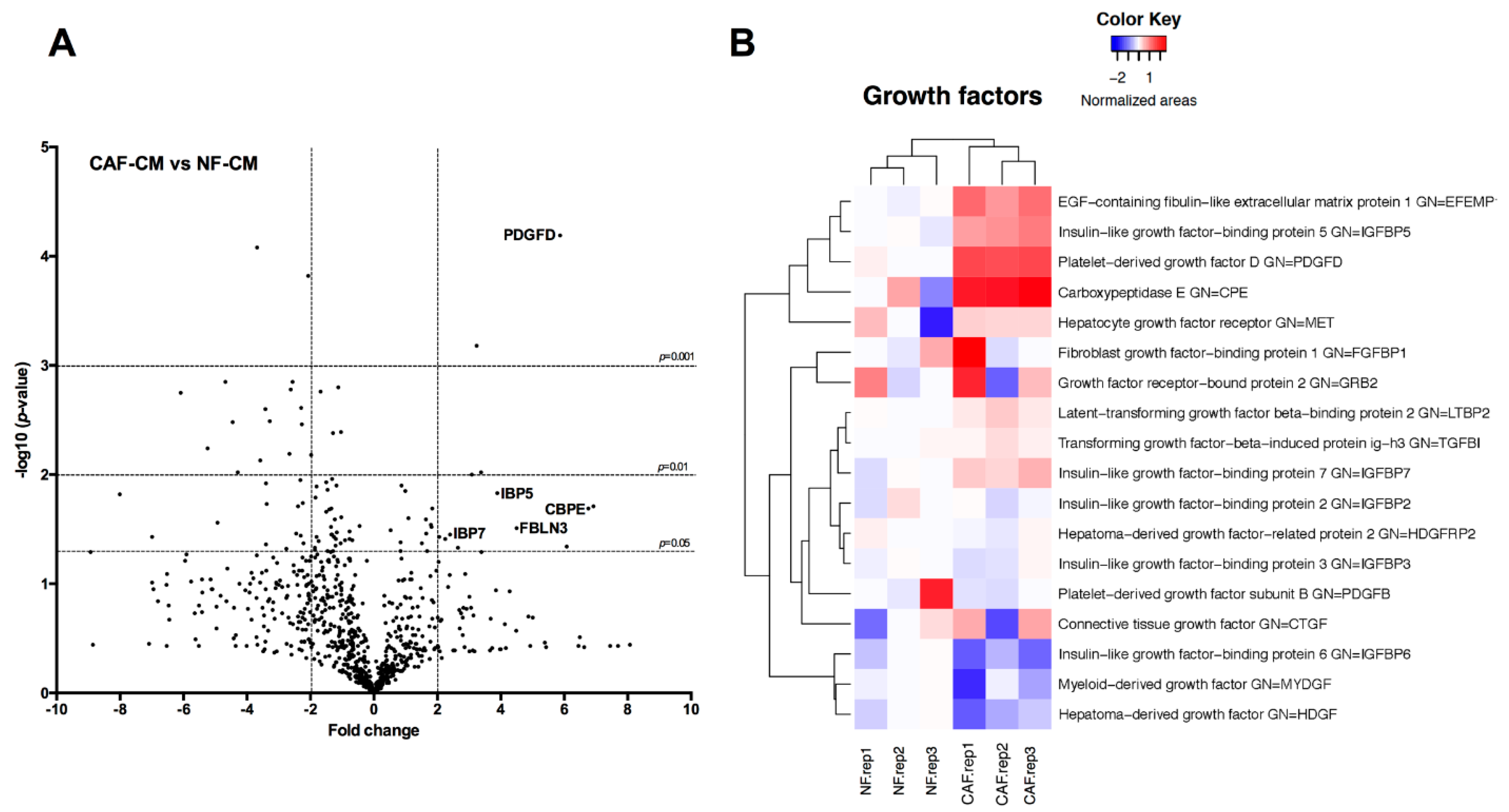 This study
investigates for the first time the crosstalk between stromal
fibroblasts and cancer stem cell (CSC) biology in head and neck squamous
cell carcinomas (HNSCC), with the ultimate goal of identifying
effective therapeutic targets. The effects of conditioned media from
cancer-associated fibroblasts (CAFs) and normal fibroblasts (NFs) on the
CSC phenotype were assessed by combining functional and expression
analyses in HNSCC-derived cell lines. Further characterization of CAFs
and NFs secretomes by mass spectrometry was followed by pharmacologic
target inhibition. We demonstrate that factors secreted by CAFs but not
NFs, in the absence of serum/supplements, robustly increased
anchorage-independent growth, tumorsphere formation, and CSC-marker
expression. Modulators of epidermal growth factor receptor (EGFR),
insulin-like growth factor receptor (IGFR), and platelet-derived growth
factor receptor (PDGFR) activity were identified as paracrine
cytokines/factors differentially secreted between CAFs and NFs, in a
mass spectrometry analysis. Furthermore, pharmacologic inhibition of
EGFR, IGFR, and PDGFR significantly reduced CAF-induced tumorsphere
formation and anchorage-independent growth suggesting a role of these
receptor tyrosine kinases in sustaining the CSC phenotype. These
findings provide novel insights into tumor stroma–CSC communication, and
potential therapeutic targets to effectively block the CAF-enhanced CSC
niche signaling circuit.
This study
investigates for the first time the crosstalk between stromal
fibroblasts and cancer stem cell (CSC) biology in head and neck squamous
cell carcinomas (HNSCC), with the ultimate goal of identifying
effective therapeutic targets. The effects of conditioned media from
cancer-associated fibroblasts (CAFs) and normal fibroblasts (NFs) on the
CSC phenotype were assessed by combining functional and expression
analyses in HNSCC-derived cell lines. Further characterization of CAFs
and NFs secretomes by mass spectrometry was followed by pharmacologic
target inhibition. We demonstrate that factors secreted by CAFs but not
NFs, in the absence of serum/supplements, robustly increased
anchorage-independent growth, tumorsphere formation, and CSC-marker
expression. Modulators of epidermal growth factor receptor (EGFR),
insulin-like growth factor receptor (IGFR), and platelet-derived growth
factor receptor (PDGFR) activity were identified as paracrine
cytokines/factors differentially secreted between CAFs and NFs, in a
mass spectrometry analysis. Furthermore, pharmacologic inhibition of
EGFR, IGFR, and PDGFR significantly reduced CAF-induced tumorsphere
formation and anchorage-independent growth suggesting a role of these
receptor tyrosine kinases in sustaining the CSC phenotype. These
findings provide novel insights into tumor stroma–CSC communication, and
potential therapeutic targets to effectively block the CAF-enhanced CSC
niche signaling circuit.